Environmental problems are created by drilling oil wells and extracting fluids because the petroleum pumped up from deep reservoir rocks is often accompanied by large volumes of salt water.
This brine contains numerous impurities, so it must either be injected back into the reservoir rocks or treated for safe surface disposal.
Petroleum usually must also be transported long distances by tanker or pipeline to reach a refinery.
Transport of petroleum occasionally leads to accidental spills.
Oil spills, especially in large volumes, can be detrimental to wildlife and habitat
Tuesday, March 10, 2009
Alternative Energy Sources
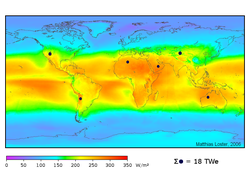
The prospect of reducing the world’s dependence on fossil fuels is problematic. Alternative energy industries, such as nuclear energy, hydroelectric energy, solar energy, wind energy, and geothermal energy exist, but these energy sources currently only account for a combined 14 percent of energy consumed worldwide. To date, alternative energy sources have been hindered by technological and environmental difficulties.
For instance, although the uranium that fuels nuclear power is abundant, the risk of nuclear accidents and the difficulty associated with safe disposal of radioactive waste have led to the decline of the nuclear power industry. Conversely, solar and wind power seem environmentally safe, but they are unreliable as steady sources of energy.
As global energy consumption grows each year, development of certain alternative energy sources becomes increasingly important. Because the global economy is powered by fossil fuels, it is critical to know how long world reserves will last. However, estimating the world’s remaining fossil fuel reserves requires extensive information, including comprehensive geological maps of the world’s sedimentary basins, models of energy production systems, and data showing world energy consumption patterns and trends.
For instance, although the uranium that fuels nuclear power is abundant, the risk of nuclear accidents and the difficulty associated with safe disposal of radioactive waste have led to the decline of the nuclear power industry. Conversely, solar and wind power seem environmentally safe, but they are unreliable as steady sources of energy.
As global energy consumption grows each year, development of certain alternative energy sources becomes increasingly important. Because the global economy is powered by fossil fuels, it is critical to know how long world reserves will last. However, estimating the world’s remaining fossil fuel reserves requires extensive information, including comprehensive geological maps of the world’s sedimentary basins, models of energy production systems, and data showing world energy consumption patterns and trends.
Fuel cell
Fuel Cell, device in which the energy of a chemical reaction is converted directly into electricity. Unlike a battery, a fuel cell does not run down; it operates as long as fuel and an oxidant are supplied continuously from outside the cell. Several companies are developing fuel cells that they hope will replace conventional internal-combustion engines in automobiles over the next few decades.
A fuel cell consists of an anode, the positive end of an electric circuit, and a cathode, the negative end of an electric circuit, separated by an electrolyte. Electrolytes are substances that allow ions (particles formed when a neutral atom or molecule gains or loses one or more electrons) to pass through them. Fuel flows to the anode, and an oxidant flows to the cathode. The chemical reaction between the fuel and the oxidant produces an electric current. Various fuels may be used, but research and development in recent years has focused on hydrogen fuel cells.
In a hydrogen fuel cell, hydrogen is supplied to the fuel cell’s anode, and an oxidant, commonly the oxygen present in air, is supplied to the cathode. The fuel cell strips electrons from the hydrogen atoms. These electrons move from the anode through the electric circuit to the cathode, creating an electric current that can be tapped to provide power.
The electron-deficient hydrogen atoms meanwhile pass through the electrolyte to the cathode. There the electrons that passed through the circuit recombine with the electron-deficient hydrogen atoms. Oxygen (from the air) reacts with this reformed hydrogen, producing water. Water produced at the cathode has to be removed continuously to avoid flooding the cell.
Hydrogen fuel cells hold great promise as low-pollution automobile engines if certain difficulties can be overcome.
Water, the only waste product of a hydrogen-oxygen fuel cell, is nonpolluting and can be used to cool the engine. The oxygen the cells need is readily available in air. Hydrogen, however, is not so readily available, and there is no existing delivery system to convey hydrogen to all the places people would need it to power their cars.
In addition, pure hydrogen is not abundant enough to provide power for all the cars on the road today. Instead, hydrogen would need to be extracted from other substances, a process that requires energy and produces pollutants.
A fuel cell consists of an anode, the positive end of an electric circuit, and a cathode, the negative end of an electric circuit, separated by an electrolyte. Electrolytes are substances that allow ions (particles formed when a neutral atom or molecule gains or loses one or more electrons) to pass through them. Fuel flows to the anode, and an oxidant flows to the cathode. The chemical reaction between the fuel and the oxidant produces an electric current. Various fuels may be used, but research and development in recent years has focused on hydrogen fuel cells.
In a hydrogen fuel cell, hydrogen is supplied to the fuel cell’s anode, and an oxidant, commonly the oxygen present in air, is supplied to the cathode. The fuel cell strips electrons from the hydrogen atoms. These electrons move from the anode through the electric circuit to the cathode, creating an electric current that can be tapped to provide power.
The electron-deficient hydrogen atoms meanwhile pass through the electrolyte to the cathode. There the electrons that passed through the circuit recombine with the electron-deficient hydrogen atoms. Oxygen (from the air) reacts with this reformed hydrogen, producing water. Water produced at the cathode has to be removed continuously to avoid flooding the cell.
Hydrogen fuel cells hold great promise as low-pollution automobile engines if certain difficulties can be overcome.
Water, the only waste product of a hydrogen-oxygen fuel cell, is nonpolluting and can be used to cool the engine. The oxygen the cells need is readily available in air. Hydrogen, however, is not so readily available, and there is no existing delivery system to convey hydrogen to all the places people would need it to power their cars.
In addition, pure hydrogen is not abundant enough to provide power for all the cars on the road today. Instead, hydrogen would need to be extracted from other substances, a process that requires energy and produces pollutants.
Labels:
Electrodes
Subscribe to:
Posts (Atom)